Luster, conductivity, and malleability are physical properties of metals., What makes these properties different from chemical properties?, A. Physical prope
SOLVED: Luster, conductivity, and malleability are physical properties of metals. What makes these properties different from chemical properties? A. Physical properties relate to elements rather than compounds. B. Physical properties appear only
Science Physics 2 Luster, conductivity, and malleability are physical properties of metals. What makes these properties different from chemical properties? Physical properties relate to elements rather than compounds. G Physical properties appear only aftera chemical change occurs. HPhysical prope tien can of the substance hnut attempting to
Source Image: letstalkscience.ca
Download Image
At the end of this metals, nonmetals, and metalloids lesson plan, students will be able to compare metals, nonmetals, and metalloids using physical properties such as luster, conductivity, or malleability. Each lesson is designed using the 5E method of instruction to ensure maximum comprehension by the students.

Source Image: classful.com
Download Image
Metals, Nonmetals and Metalloids Metals Physical Properties : Shiny Luster Malleable Ductile Conductivity Magnetism. – ppt download Sep 20, 2022The metallic bonding model explains the physical properties of metals. Metals conduct electricity and heat very well because of their free-flowing electrons. As electrons enter one end of a piece of metal, an equal number of electrons flow outward from the other end. When light is shone onto the surface of a metal, its electrons absorb small

Source Image: pinterest.com
Download Image
Compare The Physical Properties Of Luster Conductivity And Malleability
Sep 20, 2022The metallic bonding model explains the physical properties of metals. Metals conduct electricity and heat very well because of their free-flowing electrons. As electrons enter one end of a piece of metal, an equal number of electrons flow outward from the other end. When light is shone onto the surface of a metal, its electrons absorb small Teaching resources aligned to the Science TEKS for the sixth grade classroom. Including presentations, worksheet printables, projects, interactive activities, assessments, and homework materials that help teach children to compare metals, nonmetals, and metalloids using physical properties such as luster, conductivity, or malleability.
15 Properties of Metals ideas | metal, pure products, transition metal
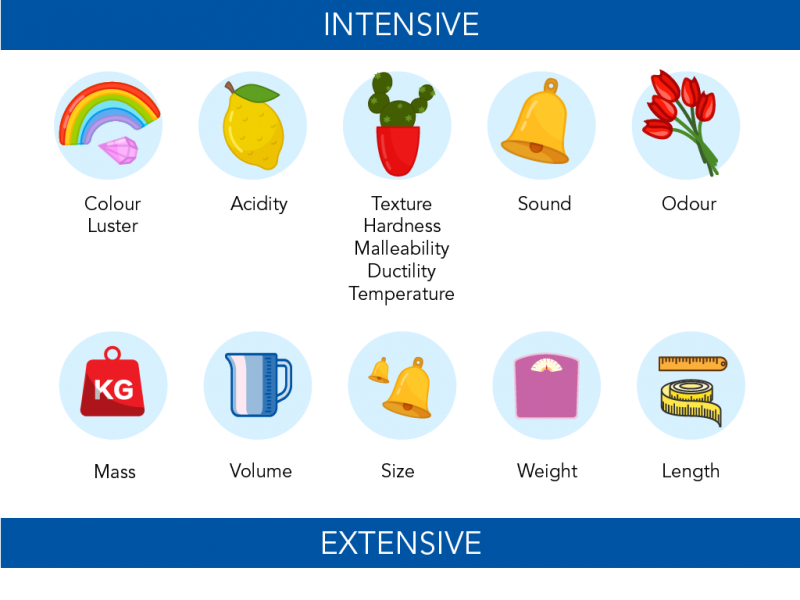
Density and temperature are intensive, when you combine 2 gallons of water the temperature stays at 20 deg (it does not become 40) and the density stays at approximately 1g/ml. Intensive properties are often constants and can be used to identify a substance. Table 1A.6. 1 1 A .6. 1: Common Physical Properties. Texture. AP 08 Ps em 04 Q A Metals and Non Metals | PDF | Metals | Silver

Source Image: scribd.com
Download Image
GotBooks.MiraCosta.edu Density and temperature are intensive, when you combine 2 gallons of water the temperature stays at 20 deg (it does not become 40) and the density stays at approximately 1g/ml. Intensive properties are often constants and can be used to identify a substance. Table 1A.6. 1 1 A .6. 1: Common Physical Properties. Texture.

Source Image: gotbooks.miracosta.edu
Download Image
SOLVED: Luster, conductivity, and malleability are physical properties of metals. What makes these properties different from chemical properties? A. Physical properties relate to elements rather than compounds. B. Physical properties appear only Luster, conductivity, and malleability are physical properties of metals., What makes these properties different from chemical properties?, A. Physical prope

Source Image: numerade.com
Download Image
Metals, Nonmetals and Metalloids Metals Physical Properties : Shiny Luster Malleable Ductile Conductivity Magnetism. – ppt download At the end of this metals, nonmetals, and metalloids lesson plan, students will be able to compare metals, nonmetals, and metalloids using physical properties such as luster, conductivity, or malleability. Each lesson is designed using the 5E method of instruction to ensure maximum comprehension by the students.

Source Image: slideplayer.com
Download Image
A Comparison of the Physical Properties of Metals and Non-Metals | PDF | Oxide | Metals Lesson objective(s): Design three testing procedures, one for each of the following: luster, conductivity, and malleability. Understand that each atom can be classified as a metal, nonmetal, or metalloid. Recognize where on the periodic table the metals, nonmetals, or metalloids are located. Categorize a given element as metal, nonmetal, or

Source Image: scribd.com
Download Image
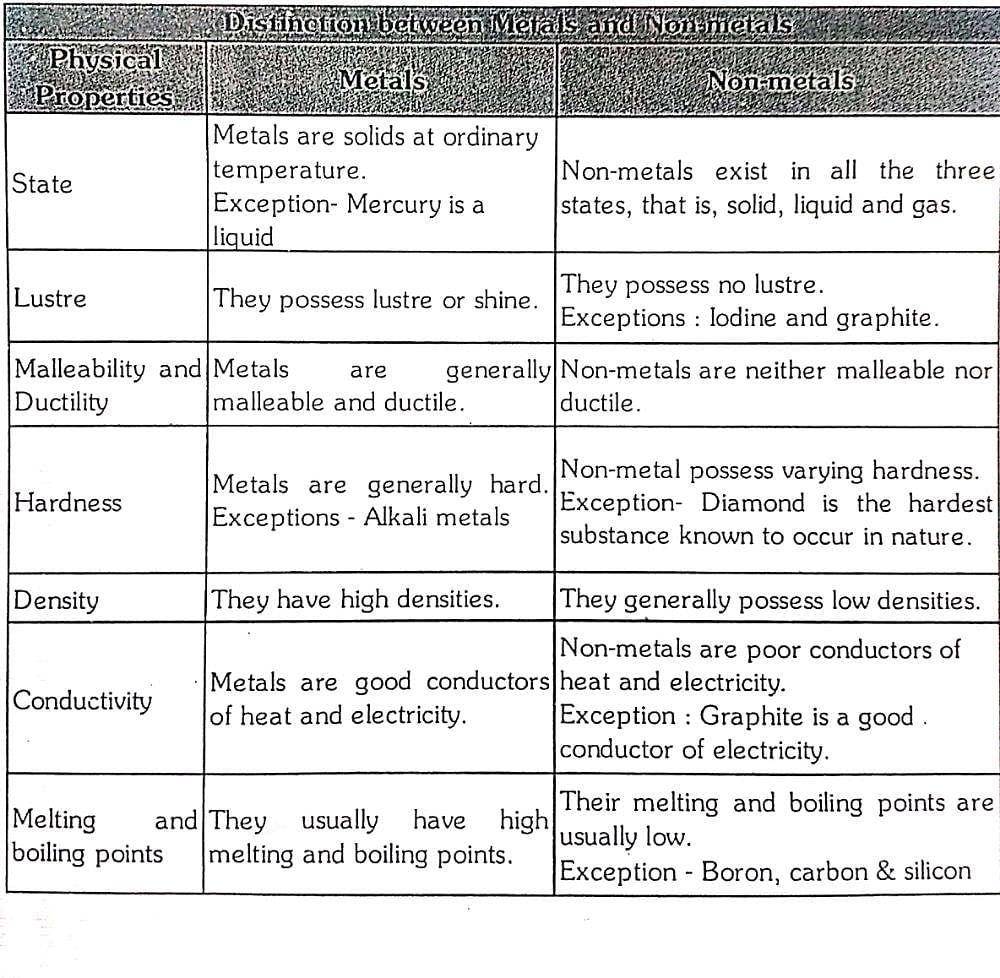
What distinction between ‘metals’and ‘non-metals’on the basis of following physical properties (a) existence (b) luster (c) hardness (d) density (e) conductivity (f) malleability and ductility ? – EduRev Class 11 Question Sep 20, 2022The metallic bonding model explains the physical properties of metals. Metals conduct electricity and heat very well because of their free-flowing electrons. As electrons enter one end of a piece of metal, an equal number of electrons flow outward from the other end. When light is shone onto the surface of a metal, its electrons absorb small
Source Image: edurev.in
Download Image
SOLUTION: Class 10 lesson 3 metals and non metaldgist of the lesson – Studypool Teaching resources aligned to the Science TEKS for the sixth grade classroom. Including presentations, worksheet printables, projects, interactive activities, assessments, and homework materials that help teach children to compare metals, nonmetals, and metalloids using physical properties such as luster, conductivity, or malleability.

Source Image: studypool.com
Download Image
GotBooks.MiraCosta.edu
SOLUTION: Class 10 lesson 3 metals and non metaldgist of the lesson – Studypool Science Physics 2 Luster, conductivity, and malleability are physical properties of metals. What makes these properties different from chemical properties? Physical properties relate to elements rather than compounds. G Physical properties appear only aftera chemical change occurs. HPhysical prope tien can of the substance hnut attempting to
Metals, Nonmetals and Metalloids Metals Physical Properties : Shiny Luster Malleable Ductile Conductivity Magnetism. – ppt download What distinction between ‘metals’and ‘non-metals’on the basis of following physical properties (a) existence (b) luster (c) hardness (d) density (e) conductivity (f) malleability and ductility ? – EduRev Class 11 Question Lesson objective(s): Design three testing procedures, one for each of the following: luster, conductivity, and malleability. Understand that each atom can be classified as a metal, nonmetal, or metalloid. Recognize where on the periodic table the metals, nonmetals, or metalloids are located. Categorize a given element as metal, nonmetal, or