If 21.4 g of aluminum is reacted with 91.3 g of FE2O3 the products will be Al2O3 and iron. What mass of iron will be Ask an Expert Answers to Homework If 21.4 g of aluminum is reacted with 91.3 g of FE2O3 the products… 11,369 Satisfied Customers B.Tech R.R. Jha is online now Related Homework Questions
SOLVED: Aluminum and oxygen react according to the chemical equation below. What mass of Al2O3 (in grams) can be made by reacting 4.6 g Al with excess oxygen? 4 Al(s) + 3
Answered step-by-step Best Matched Videos Solved By Our Top Educators If 21.4g of aluminum was reacted with 3 g iron oxide. The direction will produce lumina oxide and iron medal. The mass of iron will be produced first. The chemical equivalency needs to be written down. The react with iron oxide will produce aluminum oxide.

Source Image: pubs.acs.org
Download Image
. If 21.4 g of aluminum is reacted with 91.3 g of Fe2O3, the prodücts will be Al2O3 and iron. What mass of iron will be produced? Expert Solution Trending now This is a popular solution! Step by step Solved in 2 steps See solution Check out a sample Q&A here Knowledge Booster Learn more about Mole Concept

Source Image: pubs.acs.org
Download Image
⏩SOLVED:Metallic aluminum reacts with MnO2 at elevated temperatures… | Numerade
If 21.4 g of aluminum is reacted with 91.3 g of iron (III) oxide, the products will be aluminum oxide and iron metal. (Ar: Al=27; O=16; Fe=56) Fe2O3 + 2 Al Al2O3 + 2 Fe What mass of iron will be produced? How much non limiting reactant is left? Solution for Please give Handwritten answer.
Source Image: mdpi.com
Download Image
If 21.4 G Of Aluminum Is Reacted With
If 21.4 g of aluminum is reacted with 91.3 g of iron (III) oxide, the products will be aluminum oxide and iron metal. (Ar: Al=27; O=16; Fe=56) Fe2O3 + 2 Al Al2O3 + 2 Fe What mass of iron will be produced? How much non limiting reactant is left? Solution for Please give Handwritten answer.
Aug 31, 2023Question: If 21.4 g of aluminum is reacted with 91.3 g of Fe₂O₃, the products will be Al₂O₃ and iron. What mass of iron will be produced? Answer: 44.4 g Fe Question: If 41.6 g of N₂O₄ reacts with 20.8 g of N₂H₄, the products will be nitrogen and water. What mass of water will be produced? Answer: 23.4 g H₂O
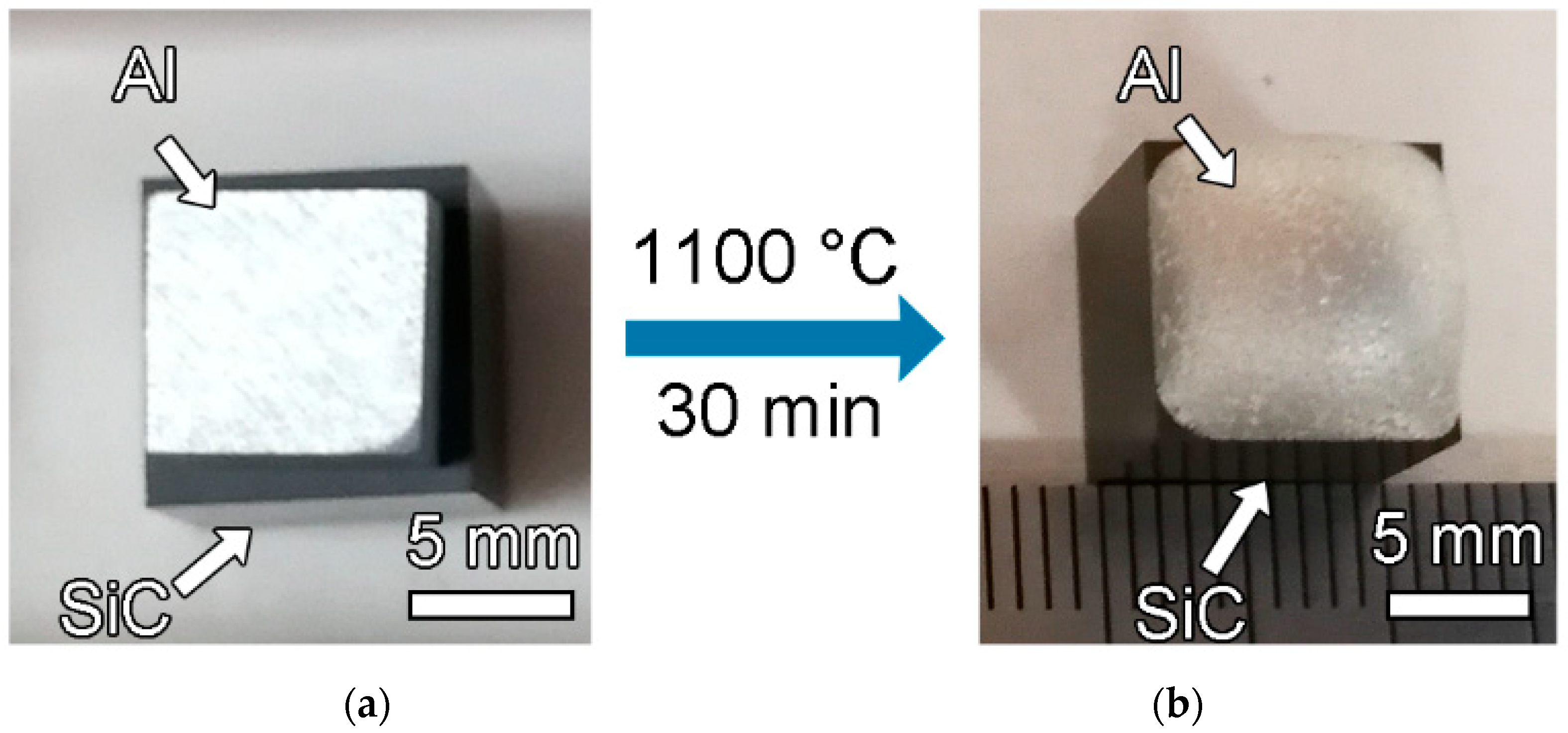
Metals | Free Full-Text | A New Strategy for Dissimilar Material Joining between SiC and Al Alloys through Use of High-Si Al Alloys
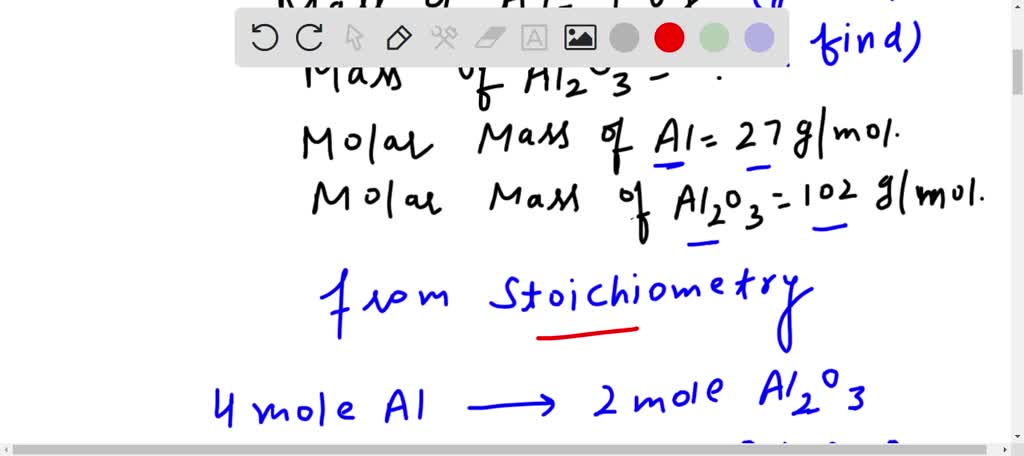
Sulfuric acid reacts with aluminum hydroxide by double replacement. a. If 30.0 g of sulfuric acid react with 25.0 g of aluminum hydroxide, identify the limiting reactant. b. Determine the mass of excess reactant remaining. c. Determine the mass of each product formed. Assume 100% yield.
SOLVED: Calorimeter A 28.4 g sample of aluminum is heated to 39.4 °C, then is placed in a calorimeter containing 50.0 g of water. The temperature of the water increases from 21.00
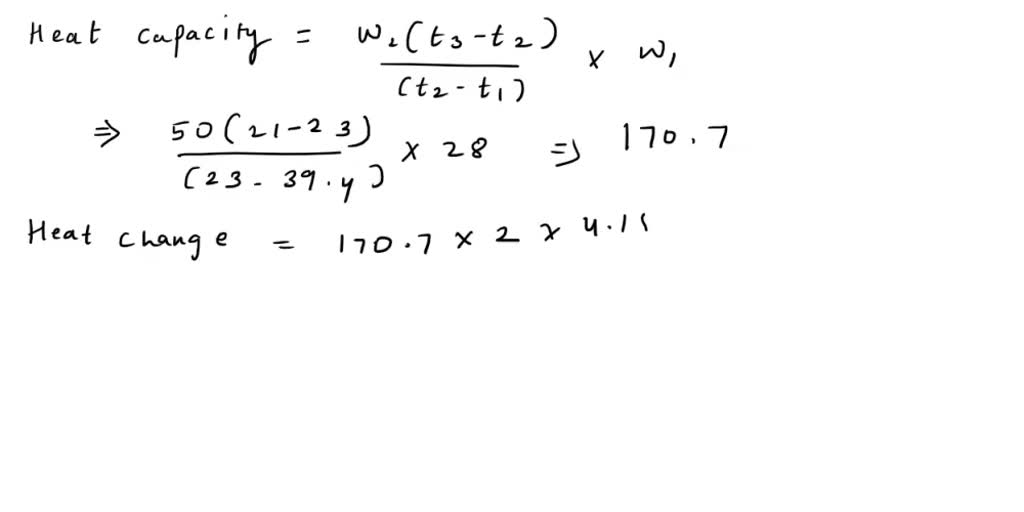
Source Image: numerade.com
Download Image
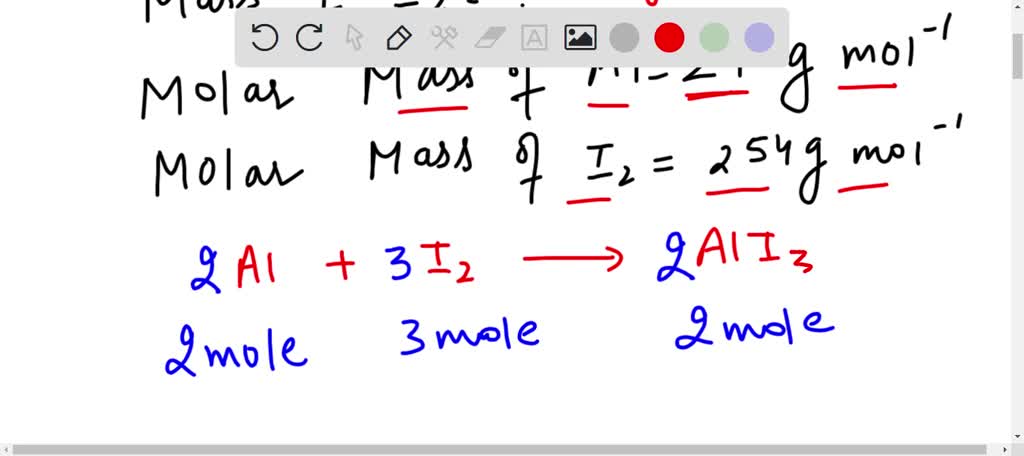
SOLVED: Calculate the mass in grams of iodine (I2) that will react completely with 20.4 g of aluminum (Al) to form aluminum iodide (AlI3).
Sulfuric acid reacts with aluminum hydroxide by double replacement. a. If 30.0 g of sulfuric acid react with 25.0 g of aluminum hydroxide, identify the limiting reactant. b. Determine the mass of excess reactant remaining. c. Determine the mass of each product formed. Assume 100% yield.
Source Image: numerade.com
Download Image
SOLVED: Aluminum and oxygen react according to the chemical equation below. What mass of Al2O3 (in grams) can be made by reacting 4.6 g Al with excess oxygen? 4 Al(s) + 3
If 21.4 g of aluminum is reacted with 91.3 g of FE2O3 the products will be Al2O3 and iron. What mass of iron will be Ask an Expert Answers to Homework If 21.4 g of aluminum is reacted with 91.3 g of FE2O3 the products… 11,369 Satisfied Customers B.Tech R.R. Jha is online now Related Homework Questions
Source Image: numerade.com
Download Image
⏩SOLVED:Metallic aluminum reacts with MnO2 at elevated temperatures… | Numerade
. If 21.4 g of aluminum is reacted with 91.3 g of Fe2O3, the prodücts will be Al2O3 and iron. What mass of iron will be produced? Expert Solution Trending now This is a popular solution! Step by step Solved in 2 steps See solution Check out a sample Q&A here Knowledge Booster Learn more about Mole Concept

Source Image: numerade.com
Download Image
⏩SOLVED:If 1.00 g of aluminum hydroxide reacts with 3.00 g of… | Numerade
Question: If 21.4 g of aluminum is reacted with 91.3 g of Fe₂O₃, the products will be Al₂O₃ and iron. What mass of iron will be produced? Answer: 44.4 g Fe Question: If 41.6 g of N₂O₄ reacts with 20.8 g of N₂H₄, the products will be nitrogen and water. What mass of water will […]

Source Image: numerade.com
Download Image
Solved Aluminum reacts with sulfur to form aluminum sulfide. | Chegg.com
If 21.4 g of aluminum is reacted with 91.3 g of iron (III) oxide, the products will be aluminum oxide and iron metal. (Ar: Al=27; O=16; Fe=56) Fe2O3 + 2 Al Al2O3 + 2 Fe What mass of iron will be produced? How much non limiting reactant is left? Solution for Please give Handwritten answer.

Source Image: chegg.com
Download Image
Oxygen | Free Full-Text | Humidity and Light Modulate Oxygen-Induced Viability Loss in Dehydrated Haematococcus lacustris Cells
Aug 31, 2023Question: If 21.4 g of aluminum is reacted with 91.3 g of Fe₂O₃, the products will be Al₂O₃ and iron. What mass of iron will be produced? Answer: 44.4 g Fe Question: If 41.6 g of N₂O₄ reacts with 20.8 g of N₂H₄, the products will be nitrogen and water. What mass of water will be produced? Answer: 23.4 g H₂O

Source Image: mdpi.com
Download Image
SOLVED: Calculate the mass in grams of iodine (I2) that will react completely with 20.4 g of aluminum (Al) to form aluminum iodide (AlI3).
Oxygen | Free Full-Text | Humidity and Light Modulate Oxygen-Induced Viability Loss in Dehydrated Haematococcus lacustris Cells
Answered step-by-step Best Matched Videos Solved By Our Top Educators If 21.4g of aluminum was reacted with 3 g iron oxide. The direction will produce lumina oxide and iron medal. The mass of iron will be produced first. The chemical equivalency needs to be written down. The react with iron oxide will produce aluminum oxide.
⏩SOLVED:Metallic aluminum reacts with MnO2 at elevated temperatures… | Numerade Solved Aluminum reacts with sulfur to form aluminum sulfide. | Chegg.com
Question: If 21.4 g of aluminum is reacted with 91.3 g of Fe₂O₃, the products will be Al₂O₃ and iron. What mass of iron will be produced? Answer: 44.4 g Fe Question: If 41.6 g of N₂O₄ reacts with 20.8 g of N₂H₄, the products will be nitrogen and water. What mass of water will […]